Explore
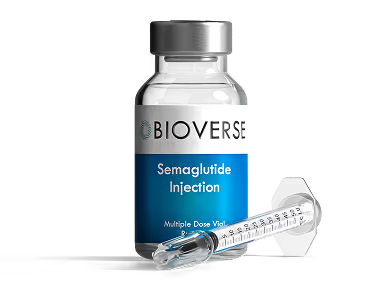
Weight Loss
NAD+ Support
Health & Longevity
Energy & Cognitive Function
Autoimmune Support
Heart Health & Blood Pressure
Glutathione Support
Erectile Dysfunction
Health Monitoring
Hair Loss
Skincare
All Treatments
Popular Treatments
Enter the Bioverse
About
FAQ












.avif)

%201.png)
.png)

